A “Down Payment” to Save Lives
By Katy Lenard, May 27, 2016
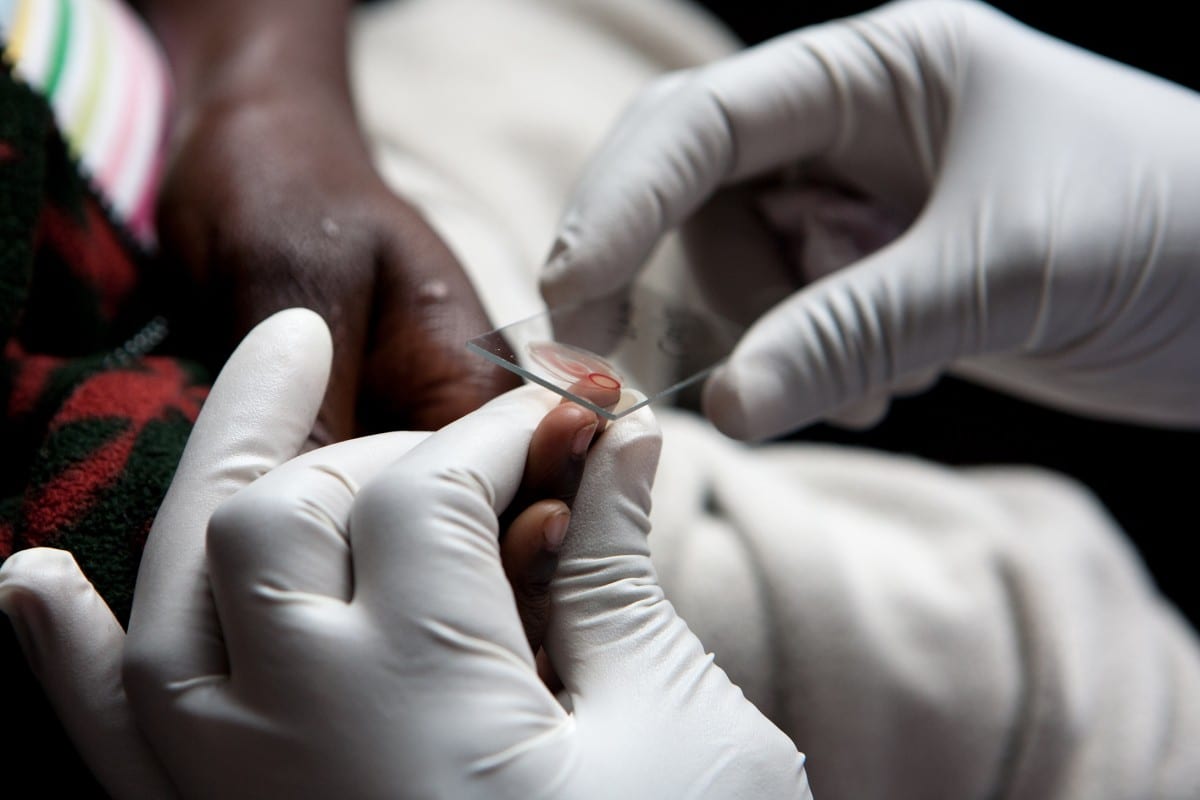
While many of us are gearing up for a long Memorial Day weekend of BBQs and beaches, President Obama is hoping that the U.S. Congress is instead preparing to make a decision, before it also goes on break, on how to fund an increasingly urgent emergency response to Zika—a devastating virus that still has no vaccine and no cure.
But according to a report from the Global Health Technologies Coalition (GHTC) launched in April on Capitol Hill, emergency funding must not be a substitute for sustained US investment in research and development (R&D) of global health technologies.
Erin Will Morton, GHTC’s coalition director, noted that the Ebola and Zika virus outbreaks have exposed to us all the perils of waiting for an emergency to trigger investment in R&D for neglected diseases. Sustained, predictable funding is not only more cost-effective, she said, it’s a down payment to save lives from the diseases we’ll face tomorrow.
The report also urged that overall underfunding for development of lifesaving tools against neglected global diseases is putting the U.S. and the world at risk. Political inaction in Washington could undermine two decades of landmark gains in global health for diseases like malaria, tuberculosis and HIV/AIDS, and also leave the world unprepared and unprotected against emerging health problems like Zika or antimicrobial resistance.
The GHTC report documents how past US support has led to breakthrough health solutions, like the first blood test for HIV/AIDS, a vaccine to prevent meningitis A in Africa, and new diagnostic tests to target drug resistant tuberculosis (TB), which have contributed significantly to major milestones in global health—since 1990, there has been a 53 percent drop in childhood deaths and a 45 percent decrease in maternal deaths.
But without sustained funding, this progress is fragile.
“It is critical not to pull back US investments now and put this arc of progress at risk,” said Morton. “As promising global health products move from laboratory testing through clinical trials, costs escalate. So with great promise and success also comes a more pronounced need for funding to see these products to the finish line.”
